What Happens When Sodium Hydroxide Reacts With Chlorine . 2 cl ̅ cl2 + 2 e ̅. A sodium atom loses an electron to a chlorine atom. 2 h2o + 2 e ̅ h2 + 2 oh ̅. the reaction of chlorine with cold sodium hydroxide solution. A chlorine atom seven electrons in the outer shell. The chlorine atom becomes a negative chloride ion. Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. The reaction between chlorine and cold dilute sodium hydroxide. revision notes on 2.3.4 the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save my. explanation of what happens when sodium reacts with chlorine: the reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties. sodium hydroxide reacts with chlorine gas in different ways according to the concentration and temperature of the solution and it gives. A sodium atom has one electron in the outer shell. The sodium atom becomes a positive sodium ion.
from www.vrogue.co
A sodium atom loses an electron to a chlorine atom. The chlorine atom becomes a negative chloride ion. explanation of what happens when sodium reacts with chlorine: revision notes on 2.3.4 the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save my. 2 h2o + 2 e ̅ h2 + 2 oh ̅. A sodium atom has one electron in the outer shell. the reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties. The reaction between chlorine and cold dilute sodium hydroxide. A chlorine atom seven electrons in the outer shell. the reaction of chlorine with cold sodium hydroxide solution.
An Aqueous Solution Of Sodium Hydroxide Reacts Comple vrogue.co
What Happens When Sodium Hydroxide Reacts With Chlorine A chlorine atom seven electrons in the outer shell. The chlorine atom becomes a negative chloride ion. sodium hydroxide reacts with chlorine gas in different ways according to the concentration and temperature of the solution and it gives. the reaction of chlorine with cold sodium hydroxide solution. 2 h2o + 2 e ̅ h2 + 2 oh ̅. the reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties. A chlorine atom seven electrons in the outer shell. The reaction between chlorine and cold dilute sodium hydroxide. Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. revision notes on 2.3.4 the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save my. A sodium atom loses an electron to a chlorine atom. 2 cl ̅ cl2 + 2 e ̅. explanation of what happens when sodium reacts with chlorine: The sodium atom becomes a positive sodium ion. A sodium atom has one electron in the outer shell.
From www.youtube.com
Reaction between calcium chloride and sodium hydroxide What Happens When Sodium Hydroxide Reacts With Chlorine A chlorine atom seven electrons in the outer shell. The sodium atom becomes a positive sodium ion. The reaction between chlorine and cold dilute sodium hydroxide. sodium hydroxide reacts with chlorine gas in different ways according to the concentration and temperature of the solution and it gives. revision notes on 2.3.4 the reactions of chlorine for the cie. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.essentialchemicalindustry.org
Chlorine What Happens When Sodium Hydroxide Reacts With Chlorine Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. A sodium atom loses an electron to a chlorine atom. the reaction of chlorine with cold sodium hydroxide solution. the reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties. explanation of. What Happens When Sodium Hydroxide Reacts With Chlorine.
From basdemax.netlify.app
28++ Hydrochloric Acid And Sodium Hydroxide Balanced Equation Basdemax What Happens When Sodium Hydroxide Reacts With Chlorine The reaction between chlorine and cold dilute sodium hydroxide. A chlorine atom seven electrons in the outer shell. Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. the reaction of chlorine with cold sodium hydroxide solution. The sodium atom becomes a positive sodium ion. The chlorine atom becomes a. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.sarthaks.com
When `Cl_(2)` gas reacts with hot and concentrated sodium hydroxide What Happens When Sodium Hydroxide Reacts With Chlorine 2 cl ̅ cl2 + 2 e ̅. A chlorine atom seven electrons in the outer shell. The sodium atom becomes a positive sodium ion. The chlorine atom becomes a negative chloride ion. The reaction between chlorine and cold dilute sodium hydroxide. 2 h2o + 2 e ̅ h2 + 2 oh ̅. A sodium atom loses an electron to. What Happens When Sodium Hydroxide Reacts With Chlorine.
From insende.netlify.app
30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende What Happens When Sodium Hydroxide Reacts With Chlorine Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. the reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties. 2 h2o + 2 e ̅ h2 + 2 oh ̅. revision notes on 2.3.4 the reactions of chlorine for the cie. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.numerade.com
SOLVEDAqueous hydrochloric acid reacts with solid sodium hydroxide to What Happens When Sodium Hydroxide Reacts With Chlorine sodium hydroxide reacts with chlorine gas in different ways according to the concentration and temperature of the solution and it gives. A chlorine atom seven electrons in the outer shell. explanation of what happens when sodium reacts with chlorine: The sodium atom becomes a positive sodium ion. A sodium atom loses an electron to a chlorine atom. A. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.numerade.com
SOLVEDWrite a balanced molecular equation for the reaction indicated What Happens When Sodium Hydroxide Reacts With Chlorine 2 h2o + 2 e ̅ h2 + 2 oh ̅. The chlorine atom becomes a negative chloride ion. sodium hydroxide reacts with chlorine gas in different ways according to the concentration and temperature of the solution and it gives. The sodium atom becomes a positive sodium ion. Because the cl2 formed at the anode and the h2 formed. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.numerade.com
SOLVED Chlorine is passed into aqueous Iron (II) Sulphate and Sodium What Happens When Sodium Hydroxide Reacts With Chlorine A chlorine atom seven electrons in the outer shell. 2 h2o + 2 e ̅ h2 + 2 oh ̅. sodium hydroxide reacts with chlorine gas in different ways according to the concentration and temperature of the solution and it gives. the reaction of chlorine with cold sodium hydroxide solution. the reaction between sodium and chlorine is. What Happens When Sodium Hydroxide Reacts With Chlorine.
From exovhomju.blob.core.windows.net
Copper (Ii) Chloride And Sodium Hydroxide Reaction at Marie Croom blog What Happens When Sodium Hydroxide Reacts With Chlorine the reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties. explanation of what happens when sodium reacts with chlorine: revision notes on 2.3.4 the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save my. A sodium atom has one electron in the. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.youtube.com
Sodium hydroxide reaction with hydrochloric acid What Happens When Sodium Hydroxide Reacts With Chlorine The chlorine atom becomes a negative chloride ion. the reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties. The sodium atom becomes a positive sodium ion. Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. revision notes on 2.3.4 the reactions. What Happens When Sodium Hydroxide Reacts With Chlorine.
From exovhomju.blob.core.windows.net
Copper (Ii) Chloride And Sodium Hydroxide Reaction at Marie Croom blog What Happens When Sodium Hydroxide Reacts With Chlorine sodium hydroxide reacts with chlorine gas in different ways according to the concentration and temperature of the solution and it gives. explanation of what happens when sodium reacts with chlorine: Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. A chlorine atom seven electrons in the outer shell.. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube What Happens When Sodium Hydroxide Reacts With Chlorine 2 cl ̅ cl2 + 2 e ̅. Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. The chlorine atom becomes a negative chloride ion. explanation of what happens when sodium reacts with chlorine: 2 h2o + 2 e ̅ h2 + 2 oh ̅. revision notes on. What Happens When Sodium Hydroxide Reacts With Chlorine.
From mammothmemory.net
Sodium reaction with hydrochloric acid is violent and quick What Happens When Sodium Hydroxide Reacts With Chlorine The reaction between chlorine and cold dilute sodium hydroxide. revision notes on 2.3.4 the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save my. 2 cl ̅ cl2 + 2 e ̅. Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must.. What Happens When Sodium Hydroxide Reacts With Chlorine.
From wisc.pb.unizin.org
4.2 Classifying Chemical Reactions Chemistry What Happens When Sodium Hydroxide Reacts With Chlorine 2 h2o + 2 e ̅ h2 + 2 oh ̅. A chlorine atom seven electrons in the outer shell. A sodium atom has one electron in the outer shell. explanation of what happens when sodium reacts with chlorine: A sodium atom loses an electron to a chlorine atom. the reaction between sodium and chlorine is a classic. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.vrogue.co
An Aqueous Solution Of Sodium Hydroxide Reacts Comple vrogue.co What Happens When Sodium Hydroxide Reacts With Chlorine the reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties. sodium hydroxide reacts with chlorine gas in different ways according to the concentration and temperature of the solution and it gives. A sodium atom loses an electron to a chlorine atom. The chlorine atom becomes a negative chloride ion. A chlorine. What Happens When Sodium Hydroxide Reacts With Chlorine.
From stock.adobe.com
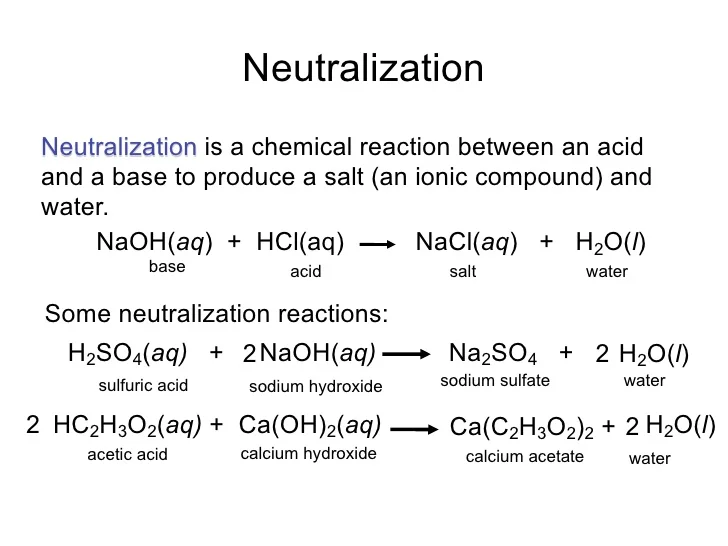
Neutralization Reaction Infographic Diagram with example of What Happens When Sodium Hydroxide Reacts With Chlorine A sodium atom has one electron in the outer shell. the reaction of chlorine with cold sodium hydroxide solution. explanation of what happens when sodium reacts with chlorine: 2 cl ̅ cl2 + 2 e ̅. revision notes on 2.3.4 the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.youtube.com
How to Balance NaOH + Cl2 = NaCl + NaClO + H2O (Dilute Sodium hydroxide What Happens When Sodium Hydroxide Reacts With Chlorine 2 h2o + 2 e ̅ h2 + 2 oh ̅. Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. the reaction between sodium and chlorine is a classic chemistry demonstration that highlights how different the properties. the reaction of chlorine with cold sodium hydroxide solution. The sodium. What Happens When Sodium Hydroxide Reacts With Chlorine.
From www.slideserve.com
PPT Reactions of chlorine with water and sodium hydroxide. PowerPoint What Happens When Sodium Hydroxide Reacts With Chlorine sodium hydroxide reacts with chlorine gas in different ways according to the concentration and temperature of the solution and it gives. Because the cl2 formed at the anode and the h2 formed at the cathode can react explosively, they must. A sodium atom has one electron in the outer shell. 2 h2o + 2 e ̅ h2 + 2. What Happens When Sodium Hydroxide Reacts With Chlorine.